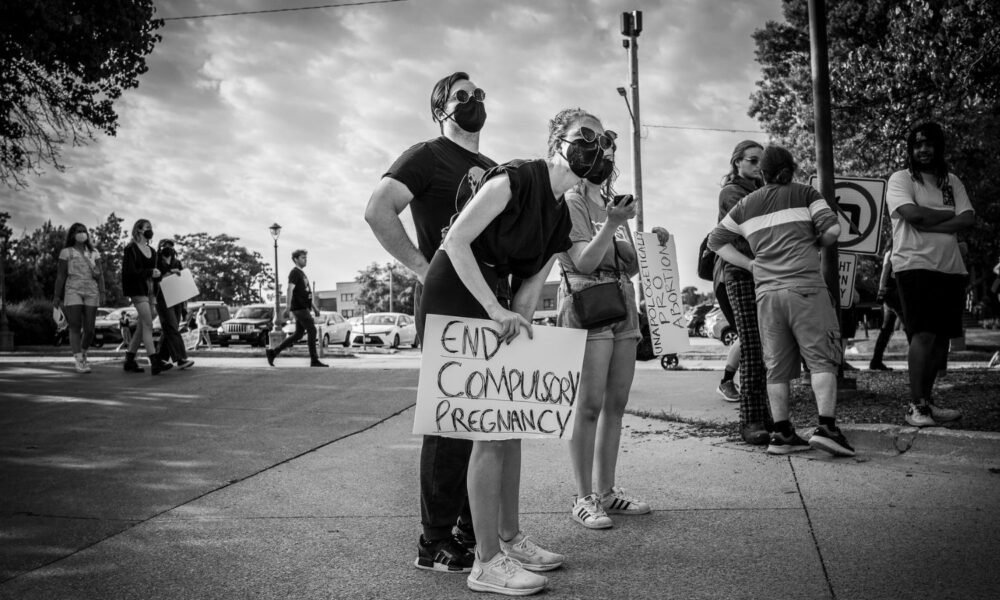This post is part of a series of quarterly roundups on scientific integrity.
During the second quarter of 2022, the Supreme Court issued decisions on abortion, gun control, and climate change regulation that ignore evidence and will harm public health. Congress and the Centers for Disease Control and Prevention failed to respond appropriately to the COVID-19 pandemic, and Congress is working on legislation that will shape the Food and Drug Administration’s oversight of drugs and devices for the next five years.
Supreme Court disregards evidence and harms public health
In some of its most consequential cases this term, the Supreme Court disregarded evidence and appeared to be motivated by politics as they handed down decisions that will be devastating for public health.
In Dobbs v. Jackson Women’s Health Organization, the Court declared that there is no constitutional right to abortion. In a decision that reverses five decades of precedent and denies a basic human right to millions, Justice Samuel Alito and his colleagues ignore extensive evidence of the harms that banning abortion will cause and the inequities it will entrench. The decision asserts that it is hard to assess “the effect of the abortion right on society and in particular on the lives of women”—but the justices received extensive evidence about those effects. High-quality research such as the University of California, San Francisco’s Turnaway Study shows that those denied wanted abortions are more likely to suffer worse outcomes years into the future, including living in poverty and continuing to experience violence from the man involved in the pregnancy. Amicus curiae briefs submitted to the Court cite this research, as well as extensive evidence that abortion bans disproportionately harm Black women (whose maternal mortality rate is more than double that of white women) and other marginalized groups, and evidence showing that abortion legalization helped women’s education, labor force participation, and earnings, with the strongest impact for Black women.
While ignoring the high-quality evidence about the impacts that abortion legalization had and the harms its removal will cause, the decision also uses problematic assertions to pretend that being forced to give birth is no big deal because some laws and benefits exist—ignoring the fact that these protect and benefit only a small share of the population and cannot erase the toll of pregnancy and childbirth, not to mention the loss of autonomy. Alito and colleagues suggest that “modern developments” make it less problematic to ban abortion. It’s true that federal and state laws ban pregnancy discrimination, but such laws have not succeeded in preventing discrimination against pregnant people. Leave for pregnancy and childbirth exists, but only about 19% of US workers have a paid family leave benefit. Yes, pregnancy-associated medical costs are covered by insurance or government assistance, but giving birth is extremely expensive even for those with private insurance—and while Medicaid might cover maternity care for those with low incomes, it won’t make up for time off work to get that care and recover after giving birth. State “safe haven” laws do let people put their newborns up for adoption, but it’s often a traumatic experience for both those relinquishing infants and the adoptees. Another problematic suggestion is that removing the federal protection from abortion rights means each state’s citizens can decide whether to ban abortion—without acknowledging that the Court has dismantled voting protections in ways that disproportionately harm communities of color.
This pattern of considering only evidence that supports a justice’s preferences for how the U.S. should function is also apparent in the New York State Rifle and Pistol Association v. Bruen decision that struck down a New York gun law—and, by extension, similar laws in other states. In this decision, Justice Clarence Thomas and his colleagues draw arbitrary lines about which historical gun laws are worth considering and which are not. “Whether a type of regulation fits within Thomas’ particular vision of ‘historical tradition,’ it turns out, is answered by whether it supports the outcome that Thomas prefers,” explains Yvette Borja of Balls and Strikes. It has not escaped notice that within the space of a week the Court decided that states cannot place limits on residents’ ability to carry guns in public without a license, but they can strip residents of the right to determine whether and when to bear children.
In West Virginia v. EPA, the Court used an obtuse interpretation of the Clean Air Act to decree that EPA does not have the authority to regulate power plants’ greenhouse-gas emissions. Using a problematic “major questions” doctrine that essentially claims agencies can’t make rules to regulate anything that the relevant law doesn’t explicitly instruct them to regulate, the Court has already blocked moves by CDC and the Occupational Safety and Health Administration (OSHA) to address the COVID-19 pandemic—not a specific hazard that those who wrote laws decades ago could have foreseen. And, as UCLA law professor Blake Emerson points out, “when Congress wrote the Administrative Procedure Act—the fundamental charter for our regulatory state—legislators repeatedly stated that agencies could decide ‘important’ decisions, so long as they did so through fair procedures.” Yet the Court ignored evidence that the people who wrote many of the laws that protect public health intended for agencies to use them to address an array of problems, not all of which were understood fully at the time of the laws’ passage.
“[A]s the Supreme Court has recognized previously, the EPA—not Congress or the courts—has the expertise to make the best policy calls based on science and technology,” note Mekela Panditharatne and Martha Kinsell of the Brennan Center for Justice. But with this decision, Vox’s Ian Milhiser explains, “The Supreme Court has effectively placed itself at the head of much of the executive branch of the federal government,” giving itself the power to strike down any regulation it dislikes using a “major questions” rationale that appears nowhere in the Constitution. As Kevin Bell of PEER observed, this ruling undermines 40 years of precedent that courts should defer to regulatory agencies on questions of statutory interpretation within their expertise, known as Chevron Deference, and is the culmination of a decades-long project by the Federalist Society and the conservative legal movement. Bell writes that the major questions doctrine will serve as “a general anesthetic to dull the thinking of the administrative state and a prohibition on comprehensive responses to emerging threats.”
These justices’ decision to ignore evidence with no apparent guiding principle except getting the majority the outcomes it wants harms the legitimacy the Supreme Court, as Justice Stephen Breyer and his colleagues warn in their dissent in the Dobbs v. JWHO decision and as a recent Gallup poll documents. It also makes it far harder to address the public health problems of climate change, gun violence, and maternal mortality, all of which disproportionately harm people who are already marginalized.
Looking ahead: Policy advocate Todd Phillips and Texas A&M University law professor Daniel E. Walters urge agencies to issue regulations aggressively rather than “clip[ping] their own wings” on the assumption that the Supreme Court will overrule them using the major questions doctrine.
Insufficient COVID-19 responses from public officials
Even as the U.S. passed the awful milestone of one million COVID-19 deaths, public officials seemed determined to downplay a pandemic that’s still causing widespread illness, death, and disability. Three months after federal funding expired for testing, treatment, and vaccinations for those without insurance, Congress has failed to renew it. As mass testing sites have closed and more people rely on at-home tests, official case counts have become an increasingly unreliable metric of the virus’s spread; by one estimate, they’re under-counting by a factor of 30.
CDC continues to highlight a “community level” map that could lull some people into a false sense of security because it focuses on whether hospitals are overwhelmed rather than on whether transmission is widespread. The transmission maps that might convince more people to take preventive steps such as wearing masks are harder to find on the agency’s website. And even where community levels are high and CDC recommends masks for indoor public places, local officials aren’t necessarily adopting mask mandates. Another loss of protections came when federal judge struck down the federal mask mandate for public transportation, a decision the Biden administration is appealing.
At a hearing on “Ensuring Scientific Integrity at Our Nation’s Public Health Agencies,” the House of Representatives’ Select Subcommittee on the Coronavirus considered a Government Accountability Office report that found career scientists observed incidents of political interference that resulted in the alteration or suppression of scientific findings related to the pandemic—but often did not report them because scientists were afraid of retaliation or suspected agency leadership was already aware of it. Anita Desikan of UCS testified about how political interference in federal science can have drastic consequences, particularly for underserved communities, and urged Congress to pass the Scientific Integrity Act to codify scientific integrity policies into law and require that all agencies implement and enforce them.
One piece of good news is that the FDA authorized both the Pfizer and Moderna COVID-19 vaccines for children aged six months to five years. The agency faced some criticism about its decision to authorize the two simultaneously, even though Moderna submitted data on its two-shot series several weeks before Pfizer submitted their three-dose data; administration officials were apparently concerned that parents who started their children on the Moderna series would be upset if the agency later authorized a Pfizer series that showed greater effectiveness. While many parents of young children celebrated the vaccine authorization news and rushed to schedule vaccination appointments, early indications suggest that the kind of racial and urban/rural inequities that we saw for other age groups could be a problem for this young age group, too.
Looking ahead: An updated booster specific to the Omicron strain of COVID-19 could be available as early as the fall.
Congress considers bills to reauthorize payments to FDA
A portion the FDA budget comes from fees paid by companies who seek agency approval for their drugs and devices, and the legislation authorizing this arrangement must be renewed every five years. Since the bill must pass, Congress adds many provisions that have nothing to do with those “user fees.” Patient, consumer, and public health groups have praised some aspects of the House and Senate versions of the bill while expressing concern about how the bills need to strengthen the scientific standards for evidence that the FDA relies on in order to safeguard public health.
A major concern is poor scientific evidence to support approval. For example, current law allows diagnostic tests produced by laboratories (rather than companies) to be sold without evidence that the tests are accurate. The Senate bill would allow the 100,000 different tests that are already on the market to remain so without evidence. It would require some evidence for a small percentage of new tests developed in the future.
“Post-market surveillance” refers to monitoring unexpected serious side effects that become apparent after a product is used by more people for longer periods of time. When the FDA approves a product based on preliminary, inconclusive data, the FDA can require companies to conduct post-market confirmatory clinical trials. This is particularly important when the FDA uses an accelerated approval pathway to approve a drug based on weak evidence because there are no other treatments for the same serious disease. The House and Senate bills would not improve the evidence for approval, but they would speed up the process of gathering confirmatory data after approval.
Patient, consumer, and public health groups urge Congressional leaders to ensure the Food and Drug Administration Safety and Landmark Advancement Act of 2022 strengthens requirements for evidence that accelerated approval drugs provide meaningful health benefits; provides resources for the FDA to improve post-market surveillance studies; and increases the diversity of the groups of patients enrolled in clinical trials. Diana Zuckerman, president of the National Center for Health Research, told STAT News that steps such as strengthening the FDA’s ability to compel manufacturers to more quickly complete research promised after accelerated approvals will help restore public trust in the agency, which lost credibility after it approved the Alzheimer’s drug Aduhelm despite a lack of evidence that it improved memory or cognitive function.
Looking ahead: Congress aims to pass the new user fee bill by September 2022, when the previous legislation will expire.
Also:
- Arati Prabhakar Set to Become Biden’s Science Adviser and His Pick to Lead Science Office
- Now Mobilizing Against Gun Violence: Scientists and Public Health Professionals
- Congress Needs to Stop Starving EPA
- Gaping Hole in Biden PFAS Strategy: Documents Show EPA Has No Consistent Definition of PFAS
- The Promise of the Environmental Justice For All Act
- The Stakes Are High for The EPA’s Newly Appointed Chemical Review Director
- A Win for Science: EPA Releases Formaldehyde Study the Chemical Industry Tried to Suppress
- ‘Pink Tape’ at the FDA is Delaying Access to Contraception — Again
- Why Some Conservative Jurists Won’t Follow the Science
New Resources:
- Center for Progressive Reform resource: A Blueprint for “Regulatory Democracy”: Empowering the Public in the Design and Implementation of New Safeguards
- EMAA Project resource: Medication Abortion Care vs. Emergency Contraception: What’s the Difference?
- Equity Forward report: In The Grand Scheme: Six Sinister Tactics Employed By Anti-Abortion Centers
- Union of Concerned Scientists resource: Countering Disinformation in Your Community