So far, we’ve provided general background on the FDA’s responsibility to ensure the safety and efficacy of prescription drugs. We have explored how the drug approval process works and examined what happens when the drug safety system breaks down. And we have looked at the importance of truly independent scientific advisory committees to the FDA’s ability to protect the public.
And today, I’d like to address three myths about how the drug approval process works in the United States–with some snazzy infographics. Read on!
Misinformation about FDA is common
Globalization has presented the FDA with new challenges in evaluating and monitoring the safety and efficacy of new medicines. Increasingly, drugs are being developed and tested overseas, and manufactured with components from multiple origins. Exacerbating this challenge is the fact that Congress has told the FDA to oversee new consumer products, such as tobacco, without sufficiently increasing the agency’s budget.
Astoundingly, Congress is considering legislative proposals that would further hamstring the FDA by reducing its authority and independence. To justify their demands, members of Congress claim that the agency hinders innovation because it is too slow and bureaucratic. They argue that the excessive oversight hurts the ability of companies to bring new drugs to market. They maintain that companies pay too much in “user fees” to have their drugs cleared for sale to the American public.
These claims are false. As Congress deliberates must-pass drug and medical device legislation, it is important to set the record straight. The information below comes from a new UCS fact sheet on the Prescription Drug User Fee Act, the law that tells FDA how to approve and monitor drugs.
Myth #1: The FDA is too slow
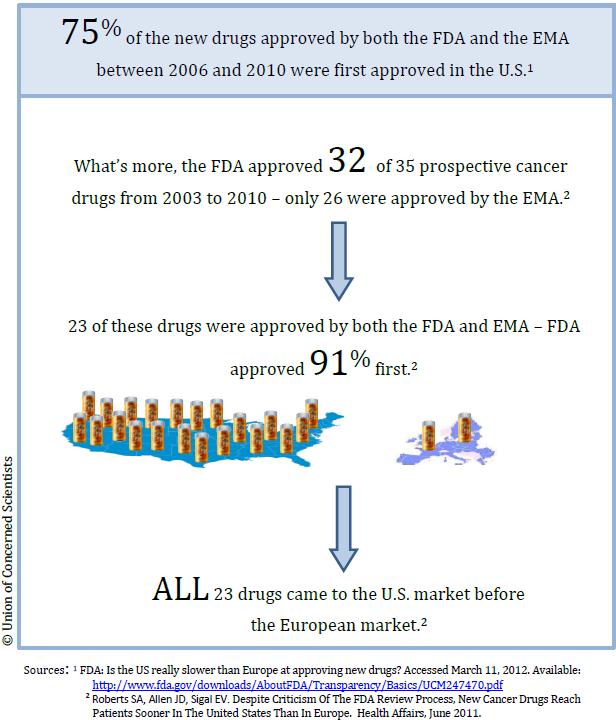
Some claim that FDA evaluates and approves drugs at a slower rate than foreign agencies such as the European Medicines Agency (EMA). In reality, the FDA was faster than its counterpart in nearly all cases:
Myth #2: The FDA has all the information it needs about the success or failure of clinical trials.
Some claim that FDA oversight of the clinical trials conducted by drug companies to evaluate the safety and effectiveness of prospective drugs is too burdensome. But companies are increasingly conducting clinical trials outside of the United States, where on-site inspections are few and far between. Furthermore, companies are more likely to report results that support approval of new products than results that do not support approval:
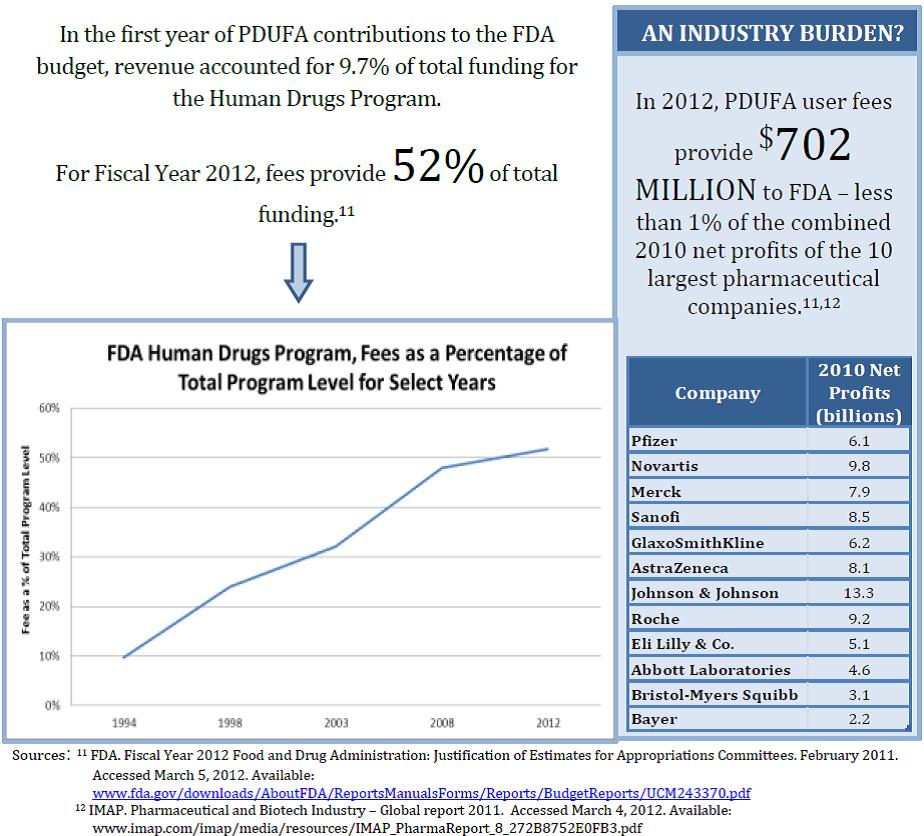
Myth #3: Drug companies pay all of the costs for FDA to review drugs and monitor their safety.
In reality, the taxpayer subsidizes the drug approval and monitoring process, even in the wake of impressive drug company profits:
Until I started working on these issues, I didn’t think much about the drug approval process. I assumed the government was taking care of it. Most of the time, it does. Most people spend little time thinking about the safety of their pharmaceutical drugs, let alone the laws and agencies overseeing the process—until something goes wrong.
The FDA’s role is to give us that peace of mind and protect us from dangerous products. We need to strengthen, not weaken, its ability to do so. And we should not fall for arguments that have no basis in fact.