Yesterday, I shared the story of a UCS supporter whose family had experienced tragic consequences when unsafe drugs were allowed on the market. But drugs and medical devices, when adequately tested and monitored, do have the potential to vastly improve one’s health. For Dan O. of Norwalk, CT, medical advances in the treatment of the coronary system have been lifesaving.
Dan and his wife Elsa have been happily married since 2007. Dan is retired from his career as a construction foreman, a job that gave him daily exercise. Elsa works as a freelancer in the publishing industry.

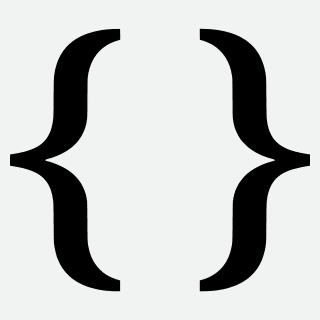
Dan and Elsa's official engagement photograph with their faithful friend. Photo: Thomas Hurlbut © 2007
Several years before they met, Dan was diagnosed with blockages in the arteries surrounding his heart. Surgeons implanted stents that open his arteries and help ensure that they function correctly, preventing heart attacks and other coronary problems.
A year and a half ago, Elsa and Dan went to the hospital for a procedure to check Dan’s stents. Although his cardiologist had ordered the procedure only as a precaution, when Dan was on the operating table the specialist informed him that scar tissue had built up around the stents, severely restricting the flow of blood through the heart.
“One of the stents was almost completely blocked,” explained Elsa over the phone. “This came as quite a surprise to both of us. We were told that this was a fairly common phenomenon, but for us it was truly frightening.”
The specialist was able to place new “drug-eluting” stents inside of Dan’s older stents; the new stents secrete a drug that discourages scar tissue from building up again (the Mayo Clinic has a brief description of different types of stents and what they do).
Stents have not been without their own controversy. Some suggest that stents are used too often, and with far too little oversight. And when a scientist published research findings that questioned the effectiveness of certain types of stents among certain populations, he was immediately attacked by the medical device industry.
But in this case, Dan may never have met Elsa if the first set of stents had not been been implanted. And it’s likely that their life together would have been cut short without the new stents.
“The experience really hit home to us,” said Elsa. “The near-blockage was located on what is commonly referred to as the ‘widowmaker’ artery. For his sake, it is critical that the makers of these types of devices are well-regulated.”
It hits home for me and my family, too. My father has drug-eluting stents in his heart. He had a check-up last Tuesday, and thankfully, the doctor told him that they appear to be working just fine.
Dan and Elsa are dedicated fans of the local Bridgeport Sound Tigers hockey team. They enjoy attending classical music concerts, and are in love with their dog nearly as much as they are in love with each other.
“We spend lots of time with our German shepherd,” Elsa told me. “We take her to sheep herding events. She’s not very good at herding, but she loves the recreation of being out in the country with the sheep.”
The type of stent used in Dan’s heart is actually a drug and a medical device. As medical products become more complex, so does the work of the FDA. For Dan and Elsa, for my dad, and for all of us, the FDA should have the resources and the independence it needs to ensure the products are safe and effective.